
Uncover the mesmerizing chemical equation for elephant toothpaste and learn how to create this foamy spectacle in a captivating science experiment.
Many of us have probably come across the mesmerizing chemical reaction known as Elephant Toothpaste. This captivating experiment involves a rapid and exothermic reaction that results in a foamy eruption resembling a giant tube of toothpaste. But have you ever wondered about the chemical equation behind this magical display? In this article, we will delve into the world of Elephant Toothpaste and uncover the secrets of its chemical equation.
Introduction to Elephant Toothpaste and its Chemical Reaction
Elephant Toothpaste is a popular experiment that never fails to amaze both children and adults alike. The reaction involves the decomposition of hydrogen peroxide (H2O2) in the presence of a catalyst, typically potassium iodide (KI), which leads to the rapid release of oxygen gas and the production of foam. This reaction is not only visually appealing but also serves as an excellent educational tool to demonstrate the principles of chemical reactions.
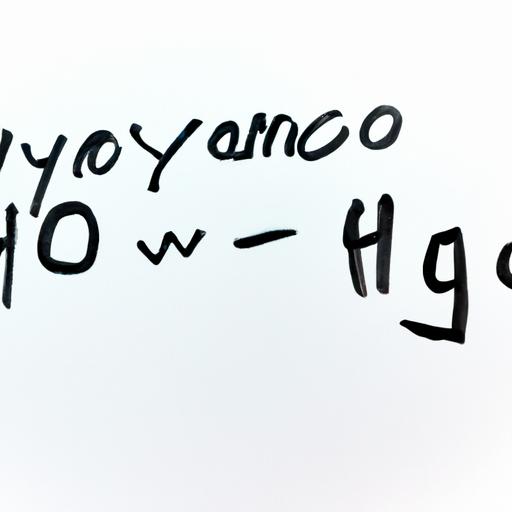
Chemical equation showcasing the decomposition of hydrogen peroxide into water and oxygen gas.
Understanding the Chemical Equation for Elephant Toothpaste
To comprehend the inner workings of this fascinating reaction, let’s take a closer look at the chemical equation involved:
2 H2O2(aq) -> 2 H2O(l) + O2(g)In this equation, hydrogen peroxide (H2O2) undergoes decomposition, breaking down into water (H2O) and oxygen gas (O2). The potassium iodide (KI) catalyst speeds up the reaction, allowing the decomposition to occur rapidly and resulting in the formation of a significant amount of foam.
Step-by-step procedure for creating Elephant Toothpaste.
Step-by-Step Procedure for Creating Elephant Toothpaste
Now that we understand the chemical equation, let’s explore how to carry out the Elephant Toothpaste experiment ourselves. Here is a step-by-step procedure to create this foamy spectacle:
-
Gather the necessary materials: You will need hydrogen peroxide (30% concentration), dish soap, food coloring (optional), a small cup or container, a funnel, a safety goggles, and gloves.
-
Prepare the setup: Place the small cup or container on a flat surface. If desired, add a few drops of food coloring to the cup to give the foam a vibrant hue. Place the funnel securely in the mouth of the container.
-
Mix the ingredients: Pour hydrogen peroxide into the cup, filling it about halfway. Add a generous squirt of dish soap into the cup and gently mix it with the hydrogen peroxide. Be cautious, as the mixture can be reactive.
-
Catalyst addition: Finally, carefully add the potassium iodide (KI) catalyst to the cup, either in solid form or as a solution, depending on the specific recipe you are following.
-
Observe the reaction: As soon as the catalyst comes in contact with the hydrogen peroxide, the chemical reaction will kick off, resulting in a massive release of oxygen gas and the formation of foam that will overflow from the container.
It’s important to note that safety precautions should be followed while conducting this experiment. Ensure you are wearing safety goggles and gloves, and perform the experiment in a well-ventilated area away from flammable substances.

Students conducting an Elephant Toothpaste experiment in a science classroom.
Applications and Importance of Elephant Toothpaste
Beyond its awe-inspiring visual appeal, Elephant Toothpaste serves as a valuable educational tool and finds practical applications in various fields. Let’s explore some of its key uses:
-
Education: Elephant Toothpaste is frequently employed in science classrooms to demonstrate the principles of chemical reactions, catalysts, and decomposition. It allows students to witness firsthand the exciting world of chemistry and encourages their curiosity.
-
Outreach and Entertainment: This experiment often takes center stage in science exhibitions, fairs, and public demonstrations due to its ability to captivate audiences of all ages. It serves as an excellent way to pique interest in science and promote scientific literacy.
-
Understanding Reaction Kinetics: Elephant Toothpaste provides a vivid illustration of the concept of reaction kinetics. By observing the rapid foam production, one can learn about the speed at which reactions occur and the factors that influence their rates.
-
Practical Applications: The knowledge gained from studying Elephant Toothpaste can be applied to various fields, including chemical engineering, environmental science, and even the development of cleaning products. Understanding the decomposition of hydrogen peroxide can lead to advancements in these areas.
Conclusion
The chemical equation for Elephant Toothpaste, 2 H2O2(aq) -> 2 H2O(l) + O2(g), unravels the mystery behind this captivating experiment. By comprehending the reaction taking place, we can create our own foamy spectacle and inspire others to explore the wonders of chemistry. Elephant Toothpaste not only entertains but also educates, making it a valuable tool in scientific outreach and learning. So, next time you witness the eruption of Elephant Toothpaste, remember the chemical equation that brings this captivating reaction to life.
Read more about toothpaste or explore the chemical equation behind Elephant Toothpaste. Discover the secrets of 3% H2O2 Elephant Toothpaste or find the perfect Elephant Toothpaste Potassium Iodide recipe for your own experiment.







